South Carolina Cervical Cancer Awareness Intitiative
Promotes statewide education and public awareness regarding cervical cancer screening and HPV vaccination cancer prevention.
DID YOU KNOW?
Get the facts you need to protect yourself from HPV-Related cancers.
Did you know that you can protect your daughter and your son from getting certain cancers?
If you are over the age of 12 you should still receive the HPV preventative vaccine.
Did you know that all women should begin cervical cancer testing at the age of 21?
Did you know that HPV and Pap screening tests offer the best chance for early cervical cancer detection?
Any male or female can "catch up" and receive the 3-dose HPV vaccine until the age of 26.
The HPV vaccine can also protect fertility in women by preventing pre-cancerous cervical lesions which sometimes lead to pregnancy loss, preterm delivery, low birthrate and other complications.
Most HPV vaccines are covered by insurance & medicaid—you can keep your child from having HPV-related cancers for free!
Both men and women can be infected by HPV.
The HPV vaccine does not lead to sexual promiscuity.
The HPV vaccine is completely safe and effective, and an important part of preventive health care.
The cost of the HPV vaccine is covered by most insurance plans and is part of Medicaid vaccines for children coverage - a small price to protect all children from preventable cancers.
HPV VACCINATION
At ages 11 through 12 years, the Advisory Committee on Immunization Practices (ACIP) recommends that preteens receive 1 dose of tetanus, diphtheria, and acellular pertussis (Tdap) vaccine, 1 dose of meningococcal conjugate (MenACWY) vaccine, a "2-dose" schedule of the human papillomavirus (HPV) vaccine for both girls AND boys, ages 9 to 14, and a "3-dose" schedule for girls AND boys 15-26 years. ACIP recommends administration of all age‐appropriate vaccines during a single visit. The Healthy People 2020 national target for vaccination coverage among adolescents by ages 13–15 years are 80.0% for ≥3 HPV doses (among females). The National Prevention Strategy includes a recommendation for health care providers to provide vaccination for HPV, as recommended by the ACIP. As of January 2014, HPV vaccination may be included as a preventive strategy as part of the Affordable Care Act.
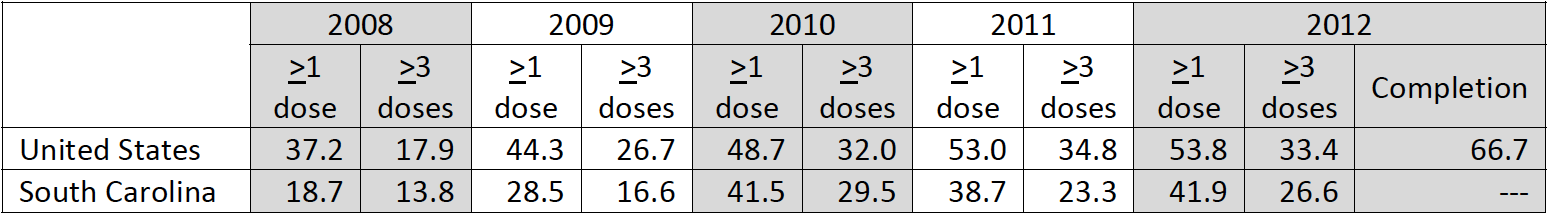
United States
– Adolescent vaccination coverage for ≥1 Tdap dose (84.6%) and ≥1 MenACWY dose (74.0%) remained higher than for
≥1 HPV vaccine dose (53.8% females; 20.8% males) in 2012.
– Among females, coverage for ≥1 HPV vaccine dose varied from 39.4% (Florida) to 73.7% (Rhode Island), and for ≥3
HPV vaccine doses, from 12.1% (Mississippi) to 57.7% (Rhode Island).
– Among males, coverage for ≥1 HPV vaccine dose ranged from 11.2% (Wyoming) to 55.2% (Rhode Island).
– Regionally, vaccination coverage was highest overall in the Northeast.
– Among males, vaccination coverage estimates for each HPV vaccine series dose and HPV series completion were
similar across regions.
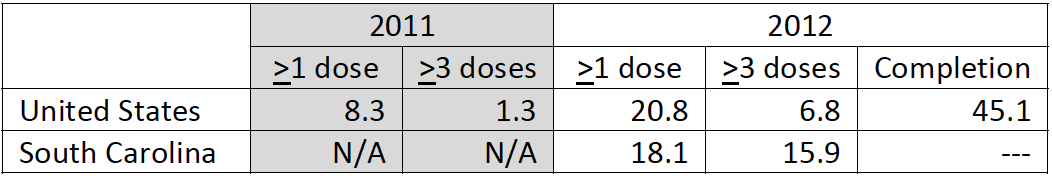
South Carolina
– Adolescent vaccination coverage for ≥1 Tdap dose (64.9%) and ≥1 MenACWY dose (58.5%) remained higher than for
≥1 HPV vaccine dose (41.9% females; 18.1% males) in 2012.
– It is important to note that vaccination coverage for Tdap and MenACWY are lower than the national average.
– Among females and males, HPV vaccination remains lower than the national average.
– Among females, coverage for ≥1 HPV vaccine dose increased from 2008‐2010, decreased in 2011, and increased to
slightly higher than the 2010 level in 2012.
– Among males, coverage for ≥1 HPV vaccine dose was 18.1% in 2012 (data unavailable in 2011).
HPV Vaccination, National Immunization Survey of 13‐17 year old teens | FEMALES (%)
HPV Vaccination, National Immunization Survey of 13‐17 year old teens | MALES (%)
BY THE NUMBERS
Facts & figures
Gardasil is approved for use in 134 countries. More than 180 million doses have been given worldwide.
´ HPV Vaccine is SAFE
´ Benefits of HPV vaccination far outweigh any potential risks
´ Safety studies findings for HPV vaccination similar to safety reviews of MCV4 and Tdap vaccination
´ Ongoing safety monitoring has shown most reports are non-serious
´ Among the 7.6% of reports coded as “serious,” most frequently cited possible side effects are headache, nausea, vomiting, and fever
´ Syncope (fainting) continues to be reported following vaccination among adolescents
´ Adherence to a 15-minute observation period after vaccination is encouraged
´ HPV Vaccine WORKS
´ Population impact against early and mid outcomes have been reported in multiple countries
´ HPV Vaccine LASTS
´ Studies suggest that vaccine protection is long-lasting
´ No evidence of waning protection
CERVICAL CANCER INCIDENCE AND MORTALITY
The Healthy People 2020 national target for cervical cancer incidence is 7.1 cases per 100,000 and for cervical cancer mortality is 2.2 deaths per 100,000. Based on 2010 data, South Carolina ranked 14th in the United States in cervical cancer incidence behind WV, OK, MS, DC, TX, LA, KS, KY, DE, TN, FL, MO and NY and 7th in cervical cancer mortality behind DE, AR, MS, WV, AL, and LA (CDC NPCR).
Table 1. Cervical Cancer Incidence, South Carolina (Total and By Race)
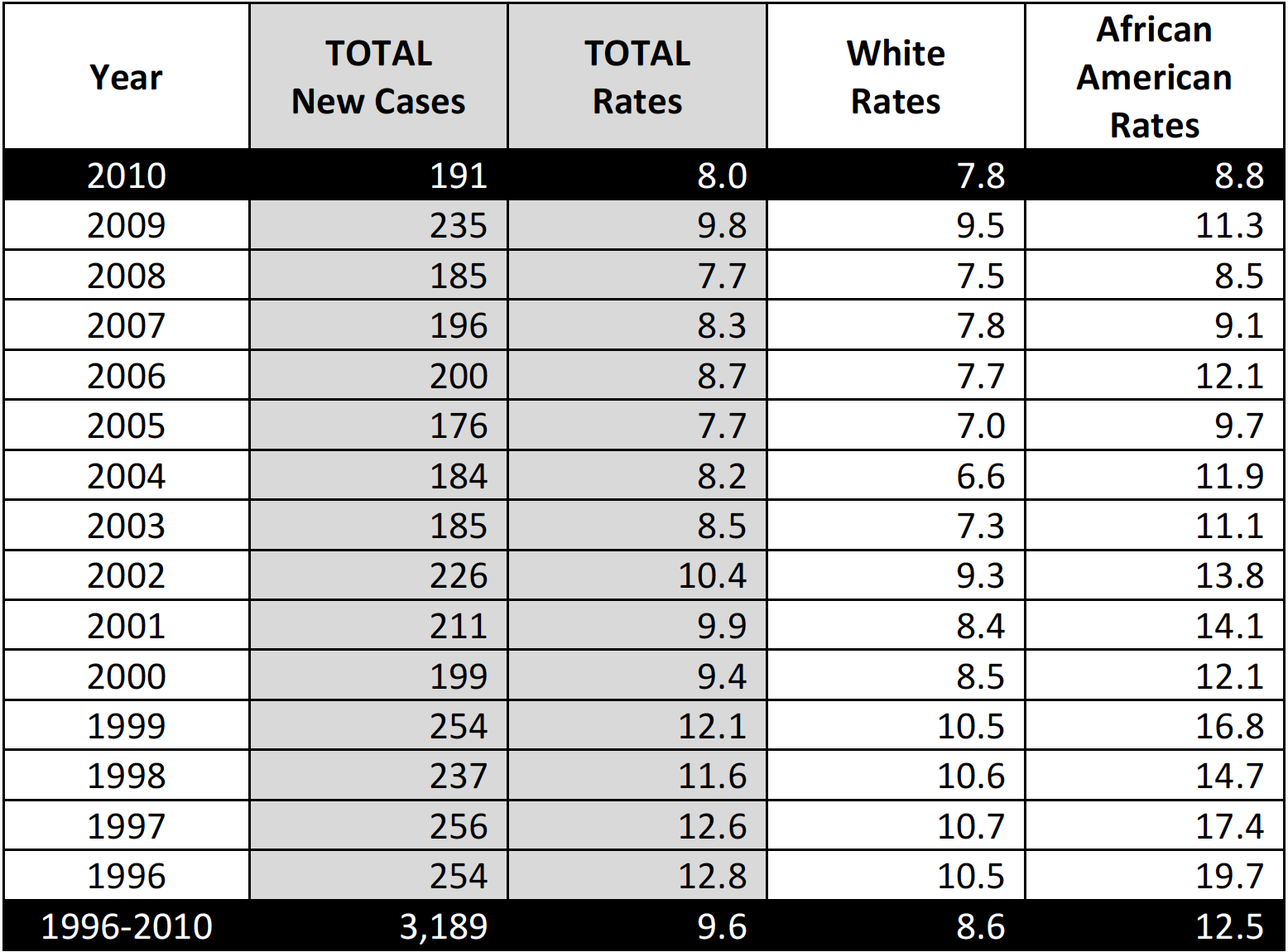
Table 2. Cervical Cancer Incidence by Age Group, South Carolina
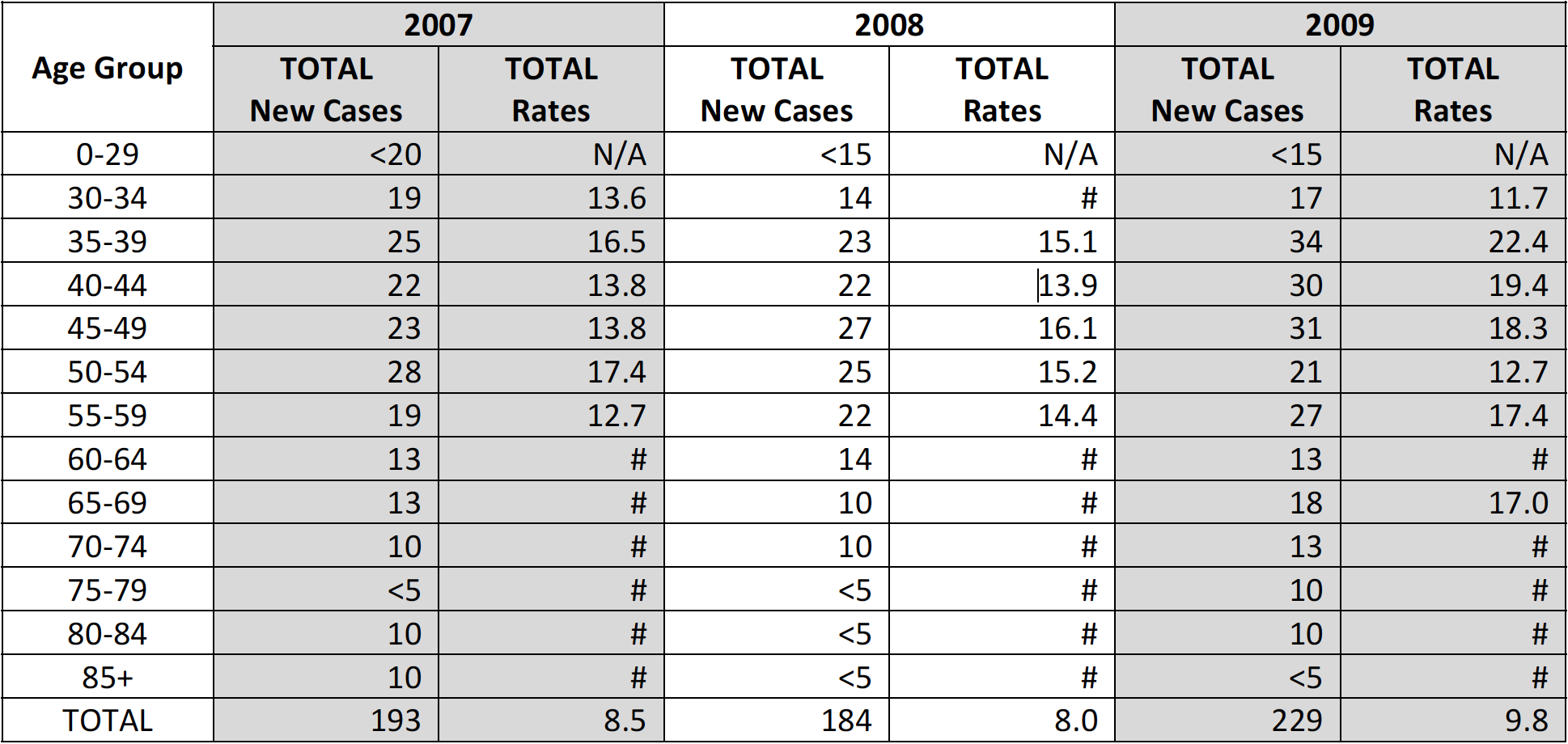
Table 3. Cervical Cancer Mortality, South Carolina (Total and By Race)
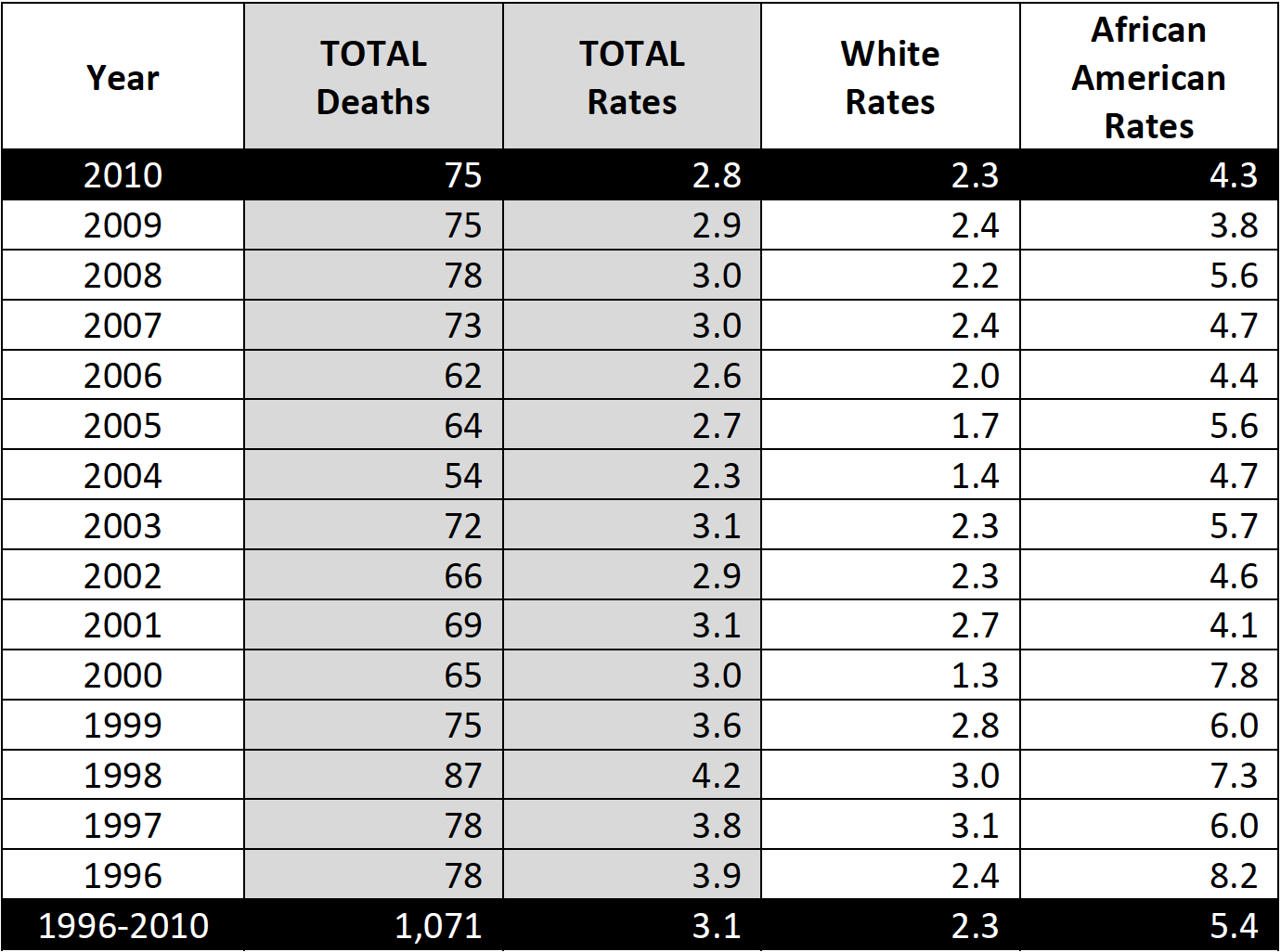
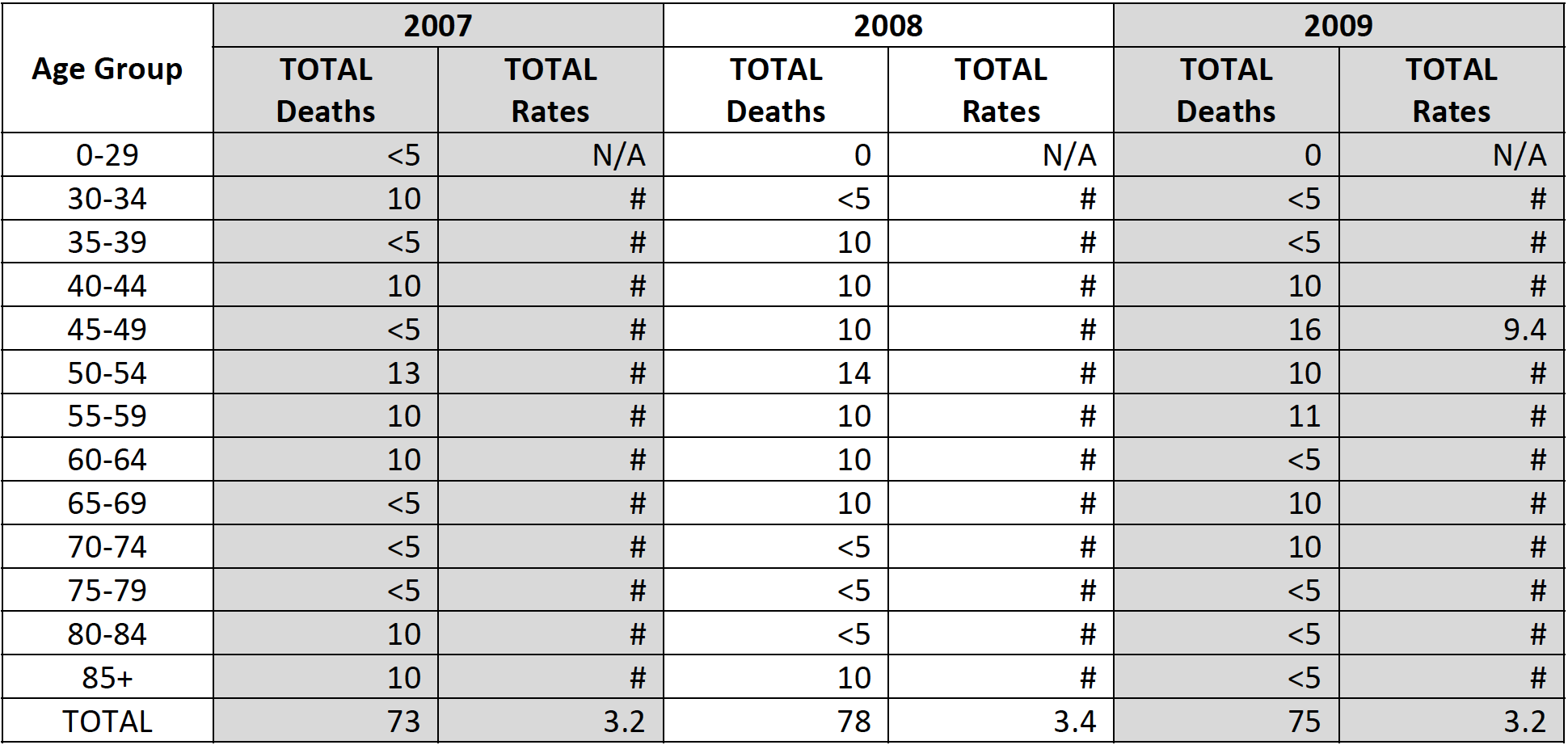
Table 4. Cervical Cancer Mortality by Age Group, South Carolina
CERVICAL CANCER SCREENING
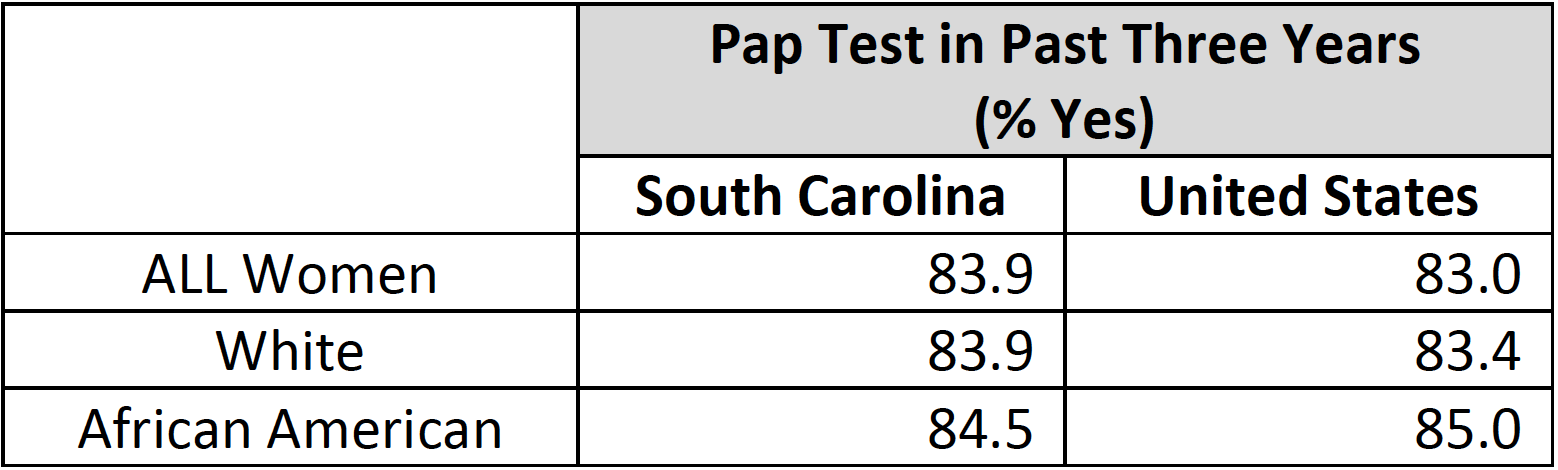
From 2000‐2010, there was a small (but significant) downward trend in cervical cancer screening in the United States and South Carolina. The Healthy People 2020 national target for cervical cancer screening is 93.0% of females aged 21 to 65 years based on the most recent guidelines.
Cervical Cancer Screening (Total and By Race)